Ventriculo-arterial coupling: From physiological concept to clinical application in peri-operative care and ICUs
Web link: Open online
Zotero link: Open in Zotero
Tags: VA coupling
Abstract
As an extension of the traditional heart-centred pressure-flow model, the ventriculo-arterial coupling concept is based on the pressure–volume relationship of the left ventricle and the vascular system. Even though ventriculo-arterial coupling has been studied in cardiology for more than 30 years, its value in clinical practice in anaesthesia and ICU remains poorly known and used. The clinical interest in ventriculo-arterial coupling is derived from its strong connection with cardiac energetics and efficiency. An alteration of ventriculo-arterial coupling is a marker of disease severity and is associated with outcome. The main categories of cardio-circulatory failures observed in ICU patients commonly exhibit alterations in ventriculo-arterial coupling with typical patterns. Furthermore, the effectiveness of usual haemodynamic treatments and interventions correlates with ventriculo-arterial coupling improvements in ICU patients. Consequently, treatment and management bundles may be proposed to specifically target the correction of ventriculo-arterial uncoupling to optimise the patients’ haemodynamic status and outcome. Restoring ventriculo-arterial coupling with treatments improves outcomes in subgroups of ICU patients. Even though ventriculo-arterial coupling evaluation cannot be considered as a part of the basic core curriculum of anaesthesiologists and ICU residents, anaesthesia and ICU practitioners must be familiarised with the clinical significance of ventriculo-arterial (un)coupling and availability of its bedside noninvasive evaluation. The understanding of ventriculo-arterial coupling may be particularly important in complex haemodynamic clinical situations.
Notes
Annotations
(9/11/2022, 9:14:37 PM)
“All compartments contribute to overall cardiovascular system homeostasis and performance, although no compartment function can be modified without altering the other.” Go to annotation (Guinot et al., 2022, p. 2)
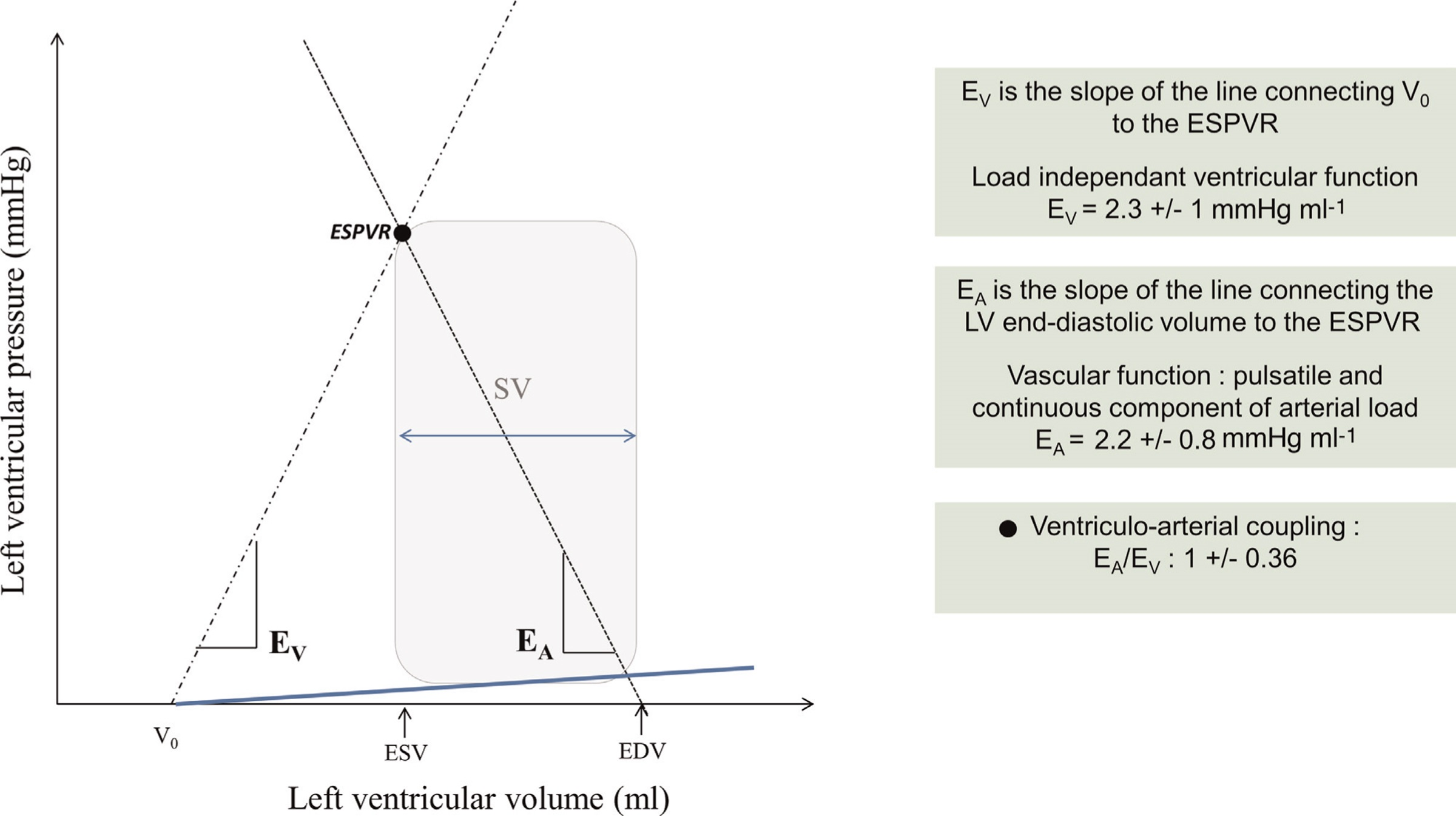
“V0 represents a theoretical left ventricular volume at zero intracavitary pressure.” Go to annotation (Guinot et al., 2022, p. 3)
“In humans, the normal value of EV is 2.3 ” Go to annotation (Guinot et al., 2022, p. 3)
“we can describe the arterial load by the slope of the relationship between the stroke volume (SV) and end-systolic arterial pressure: the arterial effective elastance (EA” Go to annotation (Guinot et al., 2022, p. 3)
“EA does not actually represent the elastance of a specific segment of the arterial tree but a cumulative parameter of the entire arterial system. EA” Go to annotation (Guinot et al., 2022, p. 3)
“In humans, the normal value of EA is 2.2 ” Go to annotation (Guinot et al., 2022, p. 3)
“In humans, the mean value of EA/EV ratio is 1 ” Go to annotation (Guinot et al., 2022, p. 3)
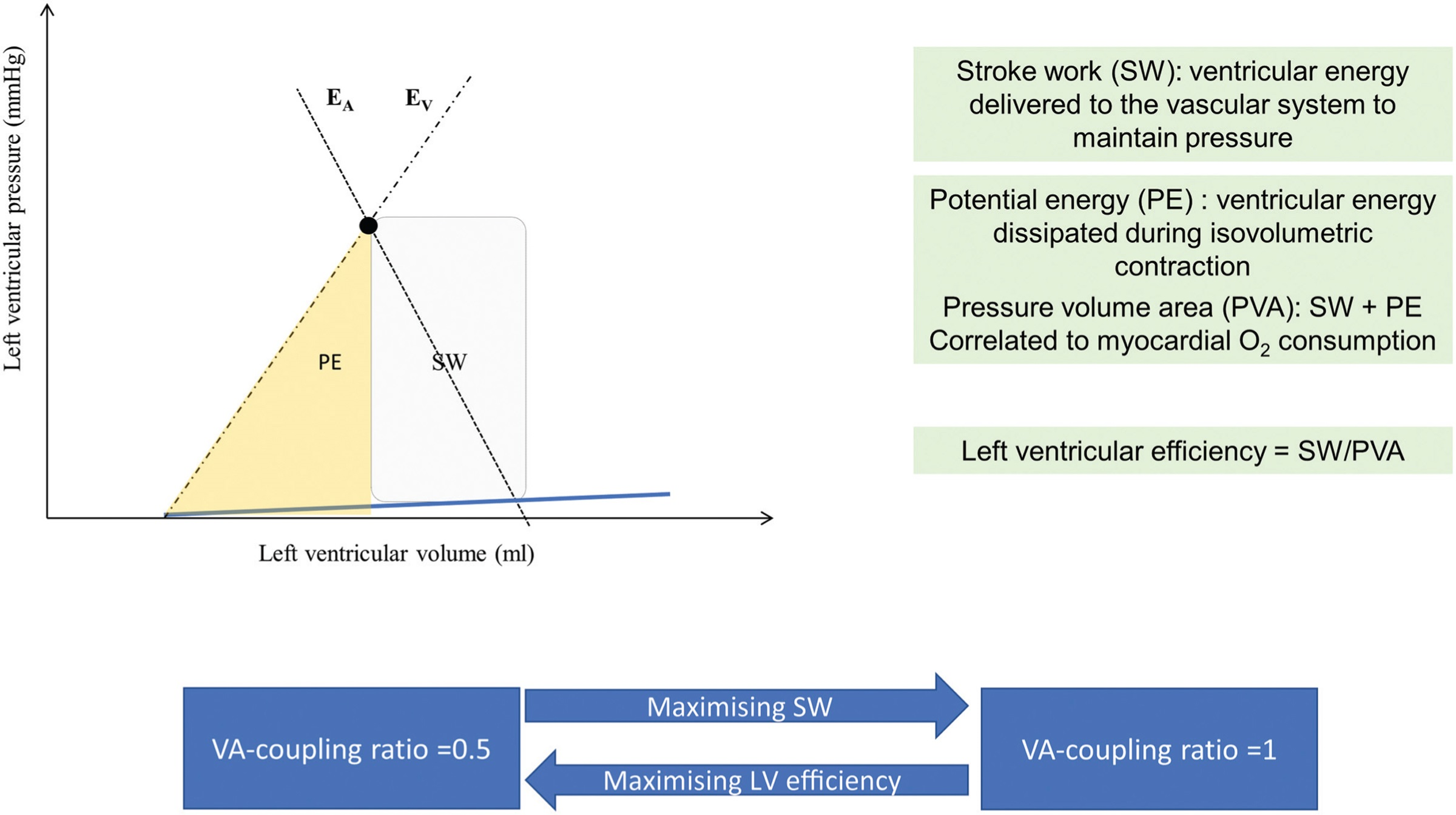
“Cardiovascular performance can not only be evaluated in terms of efficacy (blood pressure and blood flow) but also in terms of efficiency (i.e. energetic cost for the cardiovascular system to provide the same blood pressure and blood flow). Ventriculo-arterial coupling represents a clinical parameter of cardiovascular efficiency.” Go to annotation (Guinot et al., 2022, p. 4)
“The PVA is linearly correlated to myocardial oxygen consumption.13 Stroke work is the effective left ventricular energy that may be transmitted to the arterial system. Potential energy signifies the dissipated energy during the left ventricular isovolumetric contraction. Left ventricular efficiency is the ratio between stroke work and PVA.” Go to annotation (Guinot et al., 2022, p. 4)
“Left ventricular efficiency is maximised during normal physiological situations with an EA/EV” Go to annotation (Guinot et al., 2022, p. 4)
“During stressful haemodynamic situations, stroke work is maximised, reaching a maximum EA/EV ratio of 1.1 The energetic balance of the cardiovascular function is optimal when EA/EV is 1, whereas at a ratio of 0.5, maximal cardiac efficiency is observed.” Go to annotation (Guinot et al., 2022, p. 4)
“Dynamic arterial elastance (Eadyn) is the ratio of respiratory variation of pulse pressure to respiratory variation of SV.” Go to annotation (Guinot et al., 2022, p. 5)
“Pittarello et al.31 demonstrated that despite a decrease of EA and EV with remifentanil, ventriculo-arterial coupling remains unchanged. A study evaluating the effects of general anaesthesia with propofol/remifentanil infusion and positive pressure ventilation demonstrated a decrease in EV with the induction of anaesthesia and a trend for a decrease in EA; ventriculo-arterial coupling is globally unchanged.” Go to annotation (Guinot et al., 2022, p. 6)
“an increase in heart rate during exercise is associated with an increase in EV, which can match with EA in maintaining nearly optimal ventriculoarterial coupling.35 This effect is known as the forcefrequency relationship of the ventricle (the Bowditch effect), which is an intrinsic property of cardiac muscle. This effect is altered in heart failure.36 In such situations, ventriculo-arterial coupling is altered at rest, and any increase in heart rate values exacerbates ventriculo-arterial uncoupling because EA is not counterbalanced by an increase in EV.” Go to annotation (Guinot et al., 2022, p. 6)
“Acute decompensated heart failure is characterised by a high EA/EV ratio, which is caused by high EA and low EV.50 Because of low EV, patients are highly sensitive to EA, particularly through high heart rate values.51 Tachycardia, in the context of heart failure, is associated with an increase in EA because of a decrease in diastolic time and vascular compliance” Go to annotation (Guinot et al., 2022, p. 7)
“Cardiogenic shock is the most severe form of circulatory failure. It is associated with a severe decrease in EV and an initial increase and then a decrease in EA.” Go to annotation (Guinot et al., 2022, p. 7)
“Preload-dependent patients have a high baseline ventriculo-arterial coupling ratio in relation to high EA. Volume expansion improves ventriculoarterial coupling by decreasing EA, and in some cases (patients with sepsis), by increasing EV” Go to annotation (Guinot et al., 2022, p. 8)